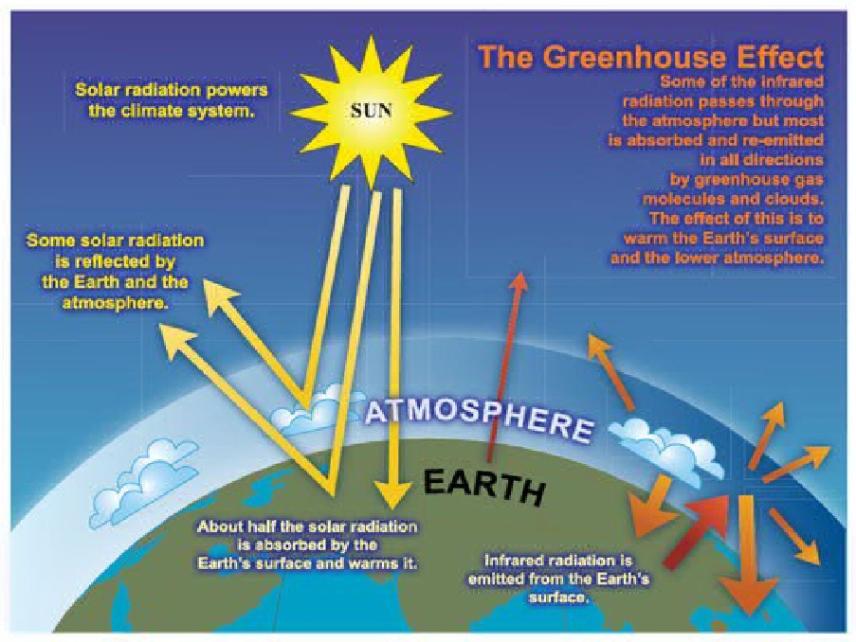
 Explain that infrared radiation is absorbed by C=O, O–H and C–H bonds in H2O, CO2 and CH4, and that these absorptions contribute to global warming; Explain that the ‘Greenhouse Effect’ of a given gas is dependent both on its atmospheric concentration and its ability to absorb infrared radiation; Outline the importance of controlling global warming resulting from atmospheric increases in greenhouse gases; Outline the role of chemists in minimising climate change resulting from global warming by:
|
||
  CH4 or methane; emitted during the production of fossil fuels , and also cows.  PROBLEMS AND HOW TO SOLVE THEM
how to solve them catalyst converters are an effective way of reducing harmful emissions from motor vehicles. chemists are minimising the climate change by
|
Carbon dioxide is produced from burning plants, volcanic eruptions and animal respiration. the concentration of carbon dioxide in the (troposphere) depends on photosynthesis, plant and animal respiration. the dissolving of carbon dioxide in the surface of waters and the quantity of carbon dioxide emitted during the combustion of fossil fuels. The absorbed energy from the infrared radiation makes the bonds vibration The Greenhouse Effect of a given gas depends on;
 Green House Effect :is the natural phenomenon by which some gases present in the atmosphere absorb infrared radiation emitted from the earth's surface and then re emitted some of this infrared radiation back to the earth's surface. the greenhouse effect is not as bad as people make it seem , because if not for the greenhouse effect the earth would of been at least 35 degrees colder than it is now. because the green house effect is natural phenomenon which keeps the earth surface warm. But there is still a problem if the green house effect increases more than the usual due to increased emisiions of carbon dioxide , CH4 and CFC's. this would lead to climate change. The green house effect of gas mainly depends on its concentration in the atmosphere and the ability it has to absorb infrared radiation.
A number of international initiatives are trying to minimse the climate change by setting targets for the reduction of their carbon dioxide emissions as of December 2006, a total of 169 countries signed up for the Kyoto Protocol. The sad news is countries like the USA and Australia refused to sign these countries are two of the biggest emitters of carbon dioxide. |
|